latent heat – salty ice
When it is icy they put salt onto the roads to melt the ice. What the salt does is to depress, or reduce, the freezing point of water. The result is that the ice on the road melts more easily. If there’s any sense in this, it means that if ice freezes at zero degrees Celsius, then salty water must freeze at an even lower temperature.

What we did
We put temperature probes into each of three ice-lolly pots in the freezer. One had 20 cm3 of water, one had 20 cm3 of salty water (brine) and one just had air. We closed the freezer door, and got a data logger to take temperature readings overnight.
The results
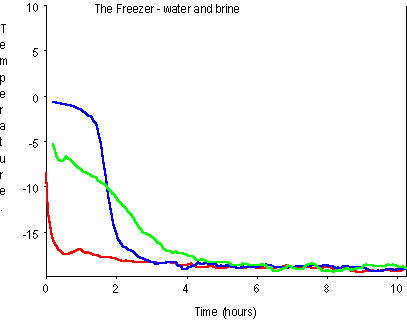
The graph shows the readings from three temperature probes: one placed in water, another in brine and another in the freezer itself. After a whole night in the freezer, the water had frozen solid but the salt water (the brine) was still slightly slushy. Here’s the graph…
The bumpy graphs are not due to a fault or sudden changes in temperature – it’s a common picture due to a mysterious thing called ‘noise’. Appreciate that these temperature probes are only supposed to work down to minus 10 degrees, so they’ve worked quite well.
Questions about the graphs (look in the zip file below for files named freezing)
You will find some of the questions easier if you import these results into Excel or your data logging software. Click here to get the results (frostfiles.zip)
- Which graph trace is which? Remember that one temperature probe was placed in water, another in brine and another in the freezer itself.
- What is the normal, steady temperature of the freezer?
- After starting the experiment and closing the door of the freezer how long did the freezer take to get back to its normal temperature?
- What does the freezing point of water appear to be? Can you explain that result?
- What can you say about the freezing point of brine?
- If you know a bit about depression of freezing point and latent heat you might be able to say why the graphs are shaped as they are.
- What you might go find out
- How is the freezing point of water affected by orange juice? What is the freezing point of ice cream? Is there any truth in the idea that oily foods do not freeze easily?